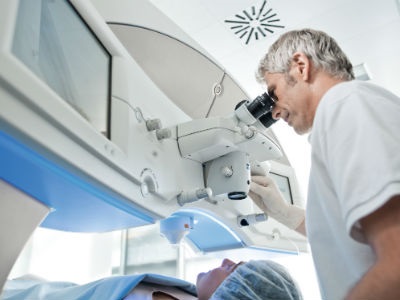
Carl Zeiss Meditec recently announced it has received U.S. FDA approval of a software update to the ZEISS VisuMax® femtosecond laser system for the ZEISS ReLEx® SMILE (SMall Incision Lenticule Extraction) procedure, and has launched a collaboration in the field of smart glasses.
With the FDA approval, surgeons can now reportedly perform SMILE for the correction of myopia in the United States. ReLEx SMILE is reportedly a minimally-invasive corneal refractive procedure that can only be performed with the ZEISS VisuMax femtosecond laser. According to Zeiss, the indication for use is in the reduction or elimination of myopia -1.00 D to -8.00 D, with ≤ -0.50 D cylinder and MRSE -8.25 D in the eye to be treated in patients who are 22 years of age or older with documentation of stable manifest refraction over the past year.
For more information, please see the press release below.
And at the recent Mobile World Congress 2017 in Barcelona, Zeiss announced a future smart glasses collaboration with Deutsche Telekom, with the goal of exploring and driving the application potential and future of data glasses.
Through one of its venture companies, Zeiss has reportedly developed a prototype of an optical system that enables a small and wearable design for data glasses, where information is projected into the wearer's field of view. Telekom would reportedly contribute direct network connection technology to this project.
Zeiss reportedly plans to release further details on this collaboration later in the year.
Click here to read the full press release.
Like what you read? Follow OphthalmologyWeb to keep up with our latest articles, news and events. Plus, get special offers and more delivered to your inbox.
Click here to read the full press release
DUBLIN, USA, March 1, 2017.
Carl Zeiss Meditec announces today that it has received U.S. FDA approval of a software update to the ZEISS VisuMax® femtosecond laser system for the ZEISS ReLEx® SMILE (SMall Incision Lenticule Extraction) procedure. With FDA approval, surgeons can now perform SMILE for the correction of myopia in the USA.
ReLEx SMILE, a minimally-invasive corneal refractive procedure which can only be performed with the ZEISS VisuMax femtosecond laser, now provides refractive surgeons in the U.S. an exciting new laser vision correction option to offer their patients. With over 700,000 procedures performed internationally since its introduction in 2011, and offered in approximately 600 clinics in 62 countries around the world, SMILE is a proven laser vision correction procedure with demonstrated safety and effectiveness.
Indication for use:
For use in the reduction or elimination of myopia -1.00 D to -8.00 D, with ≤ -0.50 D cylinder and MRSE -8.25 D in the eye to be treated in patients who are 22 years of age or older with documentation of stable manifest refraction over the past year.
Source: Carl Zeiss Meditec